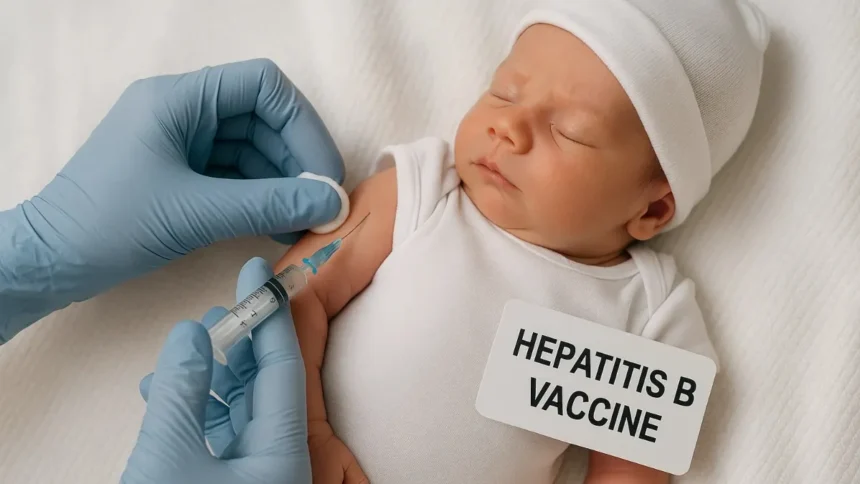
The Hepatitis B vaccine for newborns is a critical tool in preventing one of the most serious liver infections worldwide. Administered shortly after birth, this vaccine provides protection against the hepatitis B virus (HBV), which can cause chronic liver disease, cirrhosis, and liver cancer if left untreated. Over the past three decades, universal newborn vaccination has dramatically reduced HBV infections among children in the United States, underscoring its importance in public health.
The vaccine is recommended within 24 hours of birth, offering early protection to infants who may be exposed during delivery or through contact with infected individuals. Medical experts emphasize that timely vaccination not only safeguards the newborn but also reduces the long-term burden of HBV on the healthcare system.
Understanding Hepatitis B and Its Risks
Hepatitis B is a viral infection that targets the liver. It is spread through direct contact with infected blood or bodily fluids. While some adults may contract the virus through high-risk behaviors such as unprotected sex or intravenous drug use, infants are particularly vulnerable when born to HBV-positive mothers. Infections acquired during infancy are far more likely to become chronic, silently damaging the liver over years or decades.
Chronic hepatitis B often remains asymptomatic for many years, but it can progress to severe complications such as cirrhosis, liver failure, and hepatocellular carcinoma. These outcomes highlight the necessity of preventive measures, including early vaccination and ongoing public health interventions.
The History of Universal Newborn Vaccination
Before the introduction of universal vaccination, hepatitis B infections among children were relatively common, particularly in communities with higher prevalence rates. In the 1980s, public health efforts initially targeted adults at high risk for HBV, including healthcare workers, intravenous drug users, and individuals with multiple sexual partners. However, these strategies did not address the vulnerable infant population. https://www.cdc.gov/hepatitis-b/media/HepBPerinatal-ProtectHepBYourBaby.pdf
In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended a universal vaccination program for all newborns. The goal was clear: prevent vertical transmission from mother to child during birth and eliminate the early-life risk of chronic infection. Since this recommendation, cases of hepatitis B among children have declined dramatically, with reported rates in 2022 among individuals 19 years old or younger dropping to less than 0.1 per 100,000 people.
Safety and Effectiveness of the Vaccine
The Hepatitis B vaccine for newborns has an extensive safety record. Common side effects are generally mild, including slight soreness at the injection site or a low-grade fever. Severe adverse reactions are extremely rare. Multiple studies and decades of global use confirm that the vaccine is highly effective in preventing HBV infection and its long-term complications.
Medical authorities highlight that vaccinating newborns does not interfere with other routine infant immunizations. Rather, it complements the broader vaccination schedule, ensuring comprehensive protection against preventable infectious diseases.
Timing Matters: Birth Dose and Subsequent Shots
Administering the vaccine within 24 hours of birth is critical for maximum protection, especially for infants born to mothers who are HBV-positive or whose infection status is unknown. Early vaccination can prevent the virus from taking hold during the birthing process.
The typical vaccination schedule includes a birth dose followed by two or three additional doses over the first six months of life. This series ensures long-term immunity and reduces the risk of breakthrough infections. Pediatricians emphasize that delaying the birth dose could increase vulnerability, particularly in environments where HBV exposure is possible.
Protecting the Next Generation
Universal vaccination not only protects individual infants but also contributes to community-wide immunity. By preventing early-life HBV infections, the vaccine helps reduce the overall prevalence of chronic hepatitis B. This, in turn, decreases the future burden of liver disease on families and the healthcare system. Public health experts view the program as a cornerstone in the fight against liver cancer and other HBV-related complications.
Addressing Misconceptions About Newborn Vaccination
Despite the clear benefits, some parents question the need for immediate vaccination. Concerns often stem from misconceptions about vaccine safety or the perceived low risk of HBV in children. Healthcare providers emphasize that newborn vaccination is a preventive measure. Even if an infant appears healthy and unexposed, early immunization ensures protection against silent infections that could lead to lifelong complications.
Medical organizations also warn against delaying or skipping the vaccine based on non-medical reasons. Studies consistently show that early vaccination is more effective at preventing chronic infection than delayed administration.
Special Considerations for High-Risk Infants
Infants born to mothers with hepatitis B or who have unknown HBV status require special attention. In addition to the birth dose of the vaccine, these infants may receive hepatitis B immune globulin (HBIG) to provide immediate, passive protection. This combined approach offers the best chance of preventing HBV infection during the critical neonatal period.
Healthcare providers also monitor these infants closely with follow-up blood tests to ensure adequate immunity and early detection if infection occurs.
Global Perspective on Newborn Hepatitis B Vaccination
The United States is not alone in promoting early-life HBV vaccination. Countries around the world, particularly those with high HBV prevalence, have implemented similar programs. The World Health Organization (WHO) recommends that all newborns receive the first dose of hepatitis B vaccine within 24 hours of birth, followed by subsequent doses according to local guidelines.
International studies confirm that early vaccination significantly reduces the risk of chronic hepatitis B and associated liver disease, demonstrating that universal newborn vaccination is an effective and scalable public health intervention.
Ongoing Research and Vaccine Developments
Research continues to improve HBV vaccines and vaccination strategies. Scientists are investigating ways to enhance immune responses, extend the duration of protection, and simplify dosing schedules. Emerging data also support the long-term benefits of early vaccination, including lower rates of cirrhosis and hepatocellular carcinoma in adulthood.
Public health authorities remain committed to ensuring that all infants, regardless of geographic location or socioeconomic status, have access to safe and effective HBV vaccines.
Parental Guidance and Education
Educating parents about the importance of the Hepatitis B vaccine for newborns is a key component of successful immunization programs. Pediatricians provide information on vaccine safety, scheduling, and the potential consequences of HBV infection. Clear communication helps parents make informed decisions and reduces vaccine hesitancy.
Healthcare providers also emphasize that vaccination is only one component of overall child health. Routine checkups, proper nutrition, and preventive care contribute to lifelong well-being.
Conclusion
The Hepatitis B vaccine for newborns remains a cornerstone of public health, preventing serious liver disease and safeguarding future generations. Administering the vaccine within 24 hours of birth, following the recommended dosing schedule, and educating parents about its benefits ensures optimal protection. Decades of successful vaccination programs demonstrate that early intervention is not only safe and effective but essential for preventing chronic hepatitis B infections.