Gene Therapy for Huntington’s Disease: A Breakthrough in Treatment
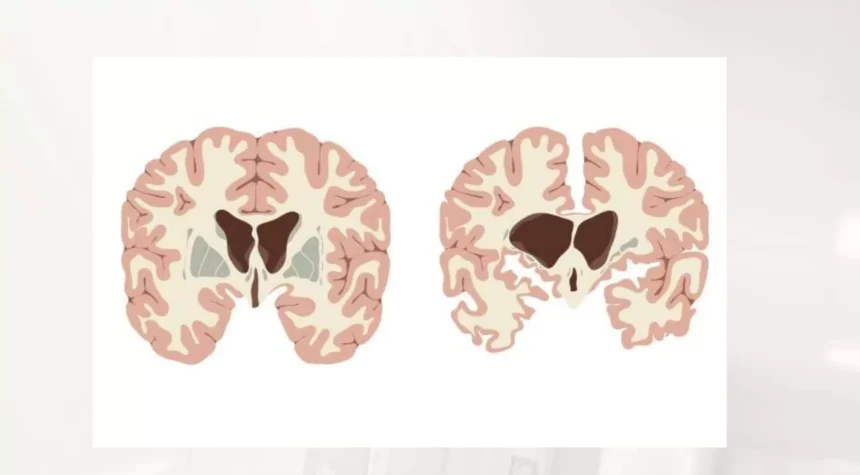
Huntington’s disease is a rare, inherited neurological disorder caused by a genetic mutation that gradually destroys nerve cells in the brain. Symptoms include involuntary movements, cognitive decline, and behavioral changes. Until recently, no treatment could slow or halt the disease’s progression. However, new developments in gene therapy are offering hope for patients and families affected by this devastating condition.
Understanding Huntington’s Disease
Huntington’s disease affects approximately 7 in 100,000 people, predominantly of European ancestry, and is less common in other populations. It typically manifests in mid-adulthood, though symptoms can appear earlier or later. The mutation responsible for Huntington’s disease causes abnormal proteins to accumulate, damaging neurons and impairing brain function.
The progressive nature of Huntington’s disease affects motor coordination, cognition, and emotional regulation. Patients often face challenges with daily activities, communication, and independence, making early intervention critical.
Breakthrough: AMT-130 Gene Therapy
A pivotal Phase 1/2 study has revealed that the experimental gene therapy AMT-130 can slow disease progression in Huntington’s patients. Administered surgically, the therapy is injected directly into the striatum, a brain region crucial for motor and cognitive functions. Patients receiving a high dose of AMT-130 experienced a 75% reduction in disease progression after 36 months.
This therapy represents one of the first potential genetic treatments for Huntington’s disease, targeting the root cause rather than merely managing symptoms.
How AMT-130 Works
AMT-130 employs a gene-silencing approach, reducing the production of harmful proteins associated with Huntington’s disease. By directly addressing the genetic mutation, the therapy may protect neurons from further damage, preserving brain function and slowing the onset of severe symptoms.
Researchers also monitored levels of neurofilament light protein, a marker of neurodegeneration, in participants’ cerebrospinal fluid. Results showed an average reduction of 8.2%, indicating the therapy’s potential effectiveness in slowing neuronal damage.
Clinical Trial Insights
The study involved 29 participants, treated with either low or high doses of AMT-130, and followed for 36 months. While the high dose showed significant benefits, the low dose did not exhibit a similar effect. Adverse events were generally related to the surgical procedure and were resolved without long-term consequences.
Assessments included motor, cognitive, behavioral, and functional tests to evaluate disease progression. These findings, though early, suggest that targeted gene therapy may transform Huntington’s treatment by addressing the underlying genetic cause.
Safety and Tolerability
Safety is a critical consideration in gene therapy, and AMT-130 was reported as “generally well-tolerated” with a manageable safety profile. Most side effects were associated with the surgical procedure itself rather than the therapy. These results provide reassurance for patients and researchers pursuing further trials. https://hdsa.org/
The Future of Huntington’s Disease Treatment
If approved by regulatory authorities, AMT-130 could become the first gene therapy available for Huntington’s disease, offering long-term benefits with a single intervention. This breakthrough highlights the potential for precision medicine to address genetic neurological disorders beyond Huntington’s disease, potentially reshaping treatment approaches for other degenerative conditions.
Implications for Patients and Families
For the estimated 42,000 Americans living with Huntington’s disease, gene therapy could provide hope for maintaining independence and improving quality of life. While the therapy is not yet widely available, clinical trials continue to expand, allowing more patients to access innovative treatments and contribute to scientific progress.
Ongoing Research and Support
Clinical trials are essential for refining gene therapy approaches and understanding long-term outcomes. Patient advocacy groups, including the Huntington’s Disease Society, emphasize cautious optimism and encourage participation in research to accelerate the development of effective therapies.
Conclusion
Gene therapy for Huntington’s disease represents a groundbreaking shift in treatment, targeting the genetic root of the disorder rather than only managing symptoms. While further research and regulatory approval are required, early findings show promise in slowing disease progression and improving patient outcomes. For those affected, gene therapy offers hope for a future where Huntington’s disease may no longer define their lives.